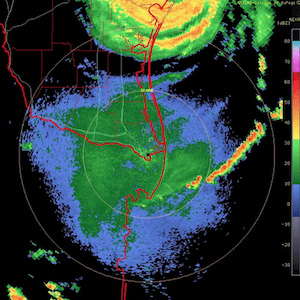
Massive spraying continues over nearly the entire Eastern Pacific. This looks like all out preparation for yet another engineered snow event which the Weather Channel will turn into theater for the coming week to convince us all we are in the grips of a ferocious winter when the truth is anything but. This ” storm” will likely be named winter storm “UKKO”. Keep an eye on the temperatures of the regions that will be impacted by this event. Some of the areas that the moisture for this storm will cross are in the upper 60 degree range currently. So how will it cold enough to snow? Artificial/chemical ice nucleation accomplished by aircraft dispersed nucleating materials which appears to be new standard for most precipitation events that have occurred this winter in the Northern Hemisphere. Watch the temps with this storm, especially as the first frozen precipitation falls. As this engineered event unfolds, the nucleation process will lower temps of the air mass to below freezing levels in some locations, when the event is over temps will be cooler though there will likely be a strong rebound of temps within a few days. Huge temperature fluctuations will continue to be the norm as geoengineering “climate forcing” devastates the climate system as a whole.
Last march shattered 15.232 high temperature records in the lower 48, with temps in Chicago in the lower 80s in mid march. The geoengineers will surely do their best to put together some lower temps in the northern tear states by jet stream manipulation and chemical ice nucleation. This will allow the Weather Channel to carry on with their cheerleading theater to convince us all of how cold it is out there. (Though 2012 was by a huge margin the warmest year ever recorded in the US.)
Heavy spraying over a great many regions is being reported. The ofter featureless “cloud” cover resulting continues to block our sunlight. “Mostly Sunny” , the term now used by most of the corporate/military/industrial/media complex weather “forecasting” agencies, generally predicts the hazy cob web like chemical “clouds” and haze from the constant spraying that now so often eliminate any semblance of blue sky.
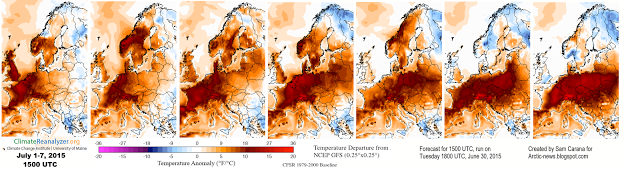
The southwest is frying, though the Weather Channel does not say much about that. Record temps pushing 100 degrees in some locations. Its going to get hotter , much hotter, and all the spraying they can carry out will only make the situation worse overall. Geoengineering is not a cure, but a curse of unimaginable proportion.
Why is the planet starting to bake? There are many reasons, including the radiant heat trapped by the spraying, but all data indicates the methane which continues spewing from the East Siberian shelf of the Arctic is the largest factor. I do not believe this issue can be kept hidden for long, as it is changing our climate and atmosphere by the day. As the Arctic ice cap disintegrates, the methane release rate will only increase. Add in the completely off the rails global spraying, along with the HAARP ionosphere heaters, and we have a recipe for a planet that will soon enough not support life if we don’t change course.
The spraying must stop, as well as the use of HAARP installations, so the planet can respond on its own, this is our best course of action.
We believe the vast majority of those that are actually carrying out the spraying do not realize what they are a part of but rather are being told they are engaged in some benevolent act for the common good. We need desperately to wake them up so they refuse to participate in these programs that are literally poisoning them and their posterity along with the rest of us.
Please help us to raise awareness till we reach critical mass, every day matters.
Dane Wigington
geoengineeringwatch.org
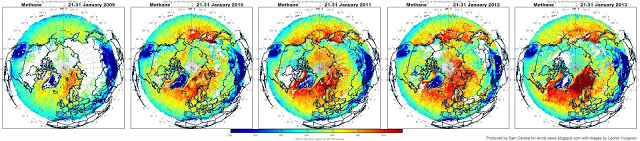
Below a combination of images showing methane levels over five years (2009 on the left, to 2013 on the right), each time for the same period (January 21-31) – images by Dr. Leonid Yurganov.
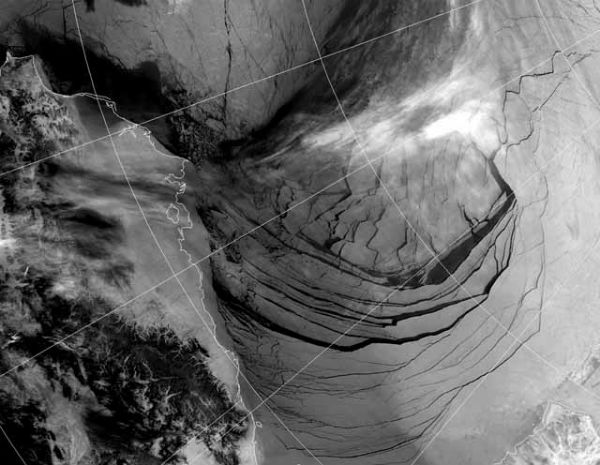
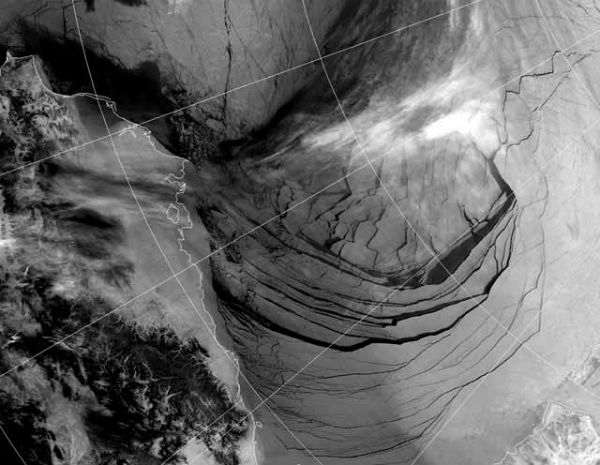
Satellite image captured the ice in the Beaufort Sea coming apart at the seams on February 27, 2013. This break up is unprecedented in the middle of the “freeze” season. We could have an “ice free” Arctic in the summer as early as this year, and likely no later than 2015. This has not happened for at least 3 million years and perhaps as long as 13 million years.



























































25 Responses
I live in Middletown, NY, about an hour northwest of New York City. On Sunday, 3/24, the first day of Spring, we saw the sun rising in a clear, blue sky. Then we saw the planes. A few hours later, the sun was completely blocked out by the oppressive, featureless total cloud cover. Again on Sunday, March 31, we awakened to a sky covered in chemtrails. The day before had been beautiful with blue sky and normal white fluffy clouds, but Sunday was scary. Monday began with a thick, heavy, unnatural fog and the sun did not show its face that day either. These are 3 days that would have normally been sunny with blue sky had they not sprayed. I suspect there are many sunny days that we have missed because of this diabolical program.
Felicia,
I live in New Windsor Ny; 35 minutes east of Middletown. Newburgh NY, New Windsor, Cornwall, etc, are located between West Point and Stewart AFB. My family and I have lived in the area for the last 20 years and we are all sick. The Chemtrails are all overand have been since I can remember. Chronic Asthma,sinus infections, fatigue, dizziness, memory loss etc.have been with me for years. My sons are Asthmatic, and experience severe allergies.
I have noticed that when I don’t go outside for an extended period of time I feel better. It is strange because last year, after going for long walks and trying to get in some exercise, I would getone bronchial infection after another.
Again, I don’t know if staying indoors( I am luckyI am able to do this) and not getting sick is a coincedence or not.
My children are adults, however, their childhood allergies and asthma are still with them.
I had read about chemtrails in the past. However, this site and others have informed me of the evil that is going on. It’s hard to believe.
http://archive.org/search.php?query=protein%20split%20products%20AND%20collection%3Aamericana alkalinity poison protein zinc corn etc!
They have part of it right look down and at the food also protein zinc alkalinity no matter what they say or do they have killed their own kids including the military protecting Monsanto etc! They have sprayed egg whites also into atmosphere! name of book on proteins early genetics stuff is Protein Split products an oldie but GOLDIE it’s for real we are at WAR!!!!!!!!!!!!!!
CHAPTER V
THE CLEAVAGE OF PROTEINS WITH DILUTE
ALKALI IN SOLUTION IN ABSOLUTE
ALCOHOL
THE researches detailed in the preceding pages seem to
establish the following propositions:
1. The cellular substances of bacteria consist largely of
proteins that yield split products identical with those
obtained by the hydrolysis of vegetable and animal proteins.
It has been shown that the bacterial cellular substances,
when broken up with mineral acids or alkalies, furnish
ammonia, mono-amino and diamino nitrogen, one or more
carbohydrate groups, and humin substances. It seemed
therefore logical to conclude that the bacterial cell consists
largely of proteins.
2. The proteins of the bacterial cell contain at least
one group which when injected intra-abdominally, subcutaneously,
or intravenously in anmials, has a markedly
poisonous effect.
3. This poisonous group may be detached from the cell
protein by hydrolysis with either dilute acids or alkalies.
4. The dilute alkali furnishes the better means of extracting
the poisonous group.
5. When the bacterial protein is broken up with alkali
in dilute aqueous solution, at least two groups are split
off and pass into solution. These are the carbohydrate
and the poisonous groups. Both are soluble in water and
in dilute alcohol, and their separation, when the cell protein
is disrupted by alkali in aqueous solution, is difficult and
unsatisfactory.
6. Since the carbohydrate group is insoluble in absolute
alcohol, while the poisonous group is more readily soluble
96 PROTEIN POISONS
in this menstruum than in water, it was decided to attempt
to disrupt the cell protein with a solution of alkali in absolute
alcohol. Another idea also acted as a determining factor
in attempting this method of hydrolysis, and in fact it
was at that time the dominating factor. The effect of the
poisonous group on animals so closely resembles that of
neurin that it was thought that the two might be identical,
or at least that the poisonous body might contain neurin.
Knowing that neurin can be heated without decomposition
in alkaline alcohol was, therefore, a reason for trying this
method.
7. Previous experiments had demonstrated the advantage
of extracting the cell substance thoroughly with alcohol and
ether before submitting it to hydrolysis. This frees the
material from fat, wax, and other substances soluble in
alcohol or ether, and since it had been shown that these
are no part of the cell protein it is beneficial to get rid of
them in toto before hydrolysis is attempted.
The following preliminary trials were made by Vaughan
and Wheeler (in the fall of 1903) in order to compare
hydrolysis with aqueous and alcoholic solutions of alkali.
Two samples, of 10 grams each, of the cellular substance
of the colon bacillus were taken. This material had previously
been thoroughly extracted with alcohol and ether.
One sample was mixed with 250 c.c. of a 1 per cent, aqueous
solution of sodium hydroxide and the other with the same
volume of an absolute alcohol solution of the same substance
in the same strength. These mixtures were heated in
flasks, fitted with reflux condensers, for one hour on the
water-bath. Ten cubic centimeters of the clear filtrate
from each was evaporated, the aqueous solution to 5 c.c.
and the alcoholic to dryness, and then taken up in 5 c.c.
of water. Each was carefully neutralized with dilute
hydrochloric acid and injected into the abdominal cavity
of a guinea-pig. Both animals developed in a characteristic
manner the first and second stages of poisoning with the
split product, but neither died. This experiment showed
that the poison was present in both extracts, and, so far
THE CLEAVAGE OF PROTEINS WITH ALKALI 97
as we could judge by the development and intensity of
the symptoms, in similar amounts. That the poison could
be extracted by alkaline alcohol was proved.
‘
However,
the yield was not satisfactory, and a second test was made,
and in this the strength of the alkali was doubled. These
were treated as before, and the pigs that received the
injections developed the characteristic symptoms and
died. The one that had the alcoholic extract died within
six, and the other within eight minutes. This confirmed
the hope that the alcoholic alkali was quite as efficient as
the aqueous in the extraction of the poisonous group.
While the aqueous extract contained a large amount of
the carbohydrate group, it was found that the alcoholic
extract, after evaporation to dryness and solution in water,
gave the biuret, Millon, and xanthoproteic tests, but failed
wholly to give the Molisch reaction. The carbohydrate
group had been split off in both samples, but being insoluble
in absolute alcohol, it remained with the insoluble portion
of the cellular substance.
The above and many other experiments have demonstrated
that the best method, so far devised, for extracting
the poisonous group from the cell protein, or, as subsequent
work has shown, from any protein, is by means of a 2 per
cent, solution of sodium’ hydroxide in absolute alcohol.
If satisfactory results are obtained, the alcohol used in the
extraction must be absolute. If it is not, more or less of
the carbohydrate will be mixed with the poison; a sticky
mass will be obtained, and the patience of the experimenter
will be taxed severely. Previous thorough extraction of
the protein with alcohol and ether for the removal of fats,
waxes, and other substances soluble in these agents, is
also essential to satisfactory work.
The method for preparing the bacterial cellular substance
has been given, but it may be well to give here some details
for the preparation of egg-white before splitting it up into
poisonous and non-poisonous proteins.
Fresh eggs (we have usually taken twenty dozen at a
time) are broken and the whites dropped into a beaker or
7
98 PROTEIN POISONS
precipitating jar, then poured with constant stirring into
four volumes of 95 per cent, alcohol. This stands with
frequent stirring for two days, then the alcohol is decanted,
and replaced with the same volume of absolute alcohol.
This is allowed to stand for from one to two days, when
the coagulated albumin is collected on a filter, allowed to
drain, then placed in large Soxhlets and extracted with
ether for from one to two days. It is then ground in porcelain
mortars and passed through fine meshed sieves. This
gives a beautifully white powder which may be kept in
bottles in stock from which portions are taken for the
purpose of hydrolyzing it.
Twenty dozen eggs yield about 735 grams of this powder,
a little more than 3 grams per egg.
A weighed portion of the protein, prepared as above, is
placed in a flask, covered with from fifteen to twenty-five
times its weight of absolute alcohol in which 2 per cent, of
sodium hydroxide has been dissolved. The flask, fitted
with a reflux condenser, is heated on the water-bath for
one hour, when it is allowed to cool and the insoluble portion
collected on a filter. After thorough draining the insoluble
part is returned to the flask and the extraction repeated.
It has been found that three extractions are necessary in
order to split off all the poisonous group. The temperature
of these extractions is 78, the temperature of boiling
absolute alcohol. By this method the protein is split into
two portions, one of which is soluble in absolute alcohol
and is poisonous, while the other is insoluble in absolute
alcohol and is not poisonous.
A large number of protein bodies, bacterial, vegetable,
and animal, have been split up in this way and no true
protein has failed to yield a poisonous portion. Among
the proteins with which we have worked the following may
be mentioned: egg-white, casein, serum albumin, edestin,
zein, Witte’s peptone, Macquaire’s peptone, de Chapoteaut’s
peptone, the tissue of cancers, and the cellular substance
of bacillus coli communis, b. typhosus, b. anthracis, b.
tuberculosis, b. Moelleri (timothy), sarcina lutea, b. ruber
THE CLEAVAGE OF PROTEINS WITH ALKALI 99
of Kiel, b. proteus, b. subtilis, b. megaterium, b. pyocyaneus,
b. pneumonise, and b. diphtheriae. Gelatin contains
no poison, but gelatin is an albuminoid and gives the
Millon test imperfectly, if at all. Nicolle and Abt1 found
that Defresne’s peptone does not yield a poison when
treated by our method, and we have confirmed this finding.
It would be interesting to know whether this peptone is
made from gelatin or from a true protein. The probabilities
are that in peptic digestion a point is reached when the
poisonous group in proteins is disrupted. In fact, as has
been stated (page 42), we have shown that the poison in
the cellular substance of the colon bacillus is slowly digested
and destroyed by digestion with pepsin-hydrochloric acid.
Therefore, it is not strange that certain peptones fail to
yield a poisonous body when disrupted with dilute alkali
in absolute alcohol. ‘ Witte’s peptone, so-called, as is well
known, is not a peptone, but an albumose.
This poison, like the whole protein of which it is a part,
is formed synthetically by the living cell. In case of the
colon poison we demonstrated this by growing the bacillus
in Fraenkel’s modification of Uschinsky’s medium, which
has the following composition:
Water 10,000 parts
Sodium chloride 50 parts
Asparagin 34 parts
Ammonium lactate 63 parts
Di-sodium hydrogen phosphate 20 parts
After a week’s development the contents of these flasks
were poured into from two to three volumes of 95 per cent,
alcohol. The precipitate was filtered out and put into
absolute alcohol; next it was extracted in Soxhlets with
ether, dried, and powdered. This powdered cellular substance,
when split up with 2 per cent, sodium hydroxide
in absolute alcohol, furnished the poison, the action of
which was demonstrated on guinea-pigs. Moreover, the
poison obtained in this way gave all the protein reactions
1 Annales de 1’Institut Pasteur, February, 1908.
100 PROTEIN POISONS
hereafter described as being obtained from the poison from
agar-grown cultures. This demonstrates that the poison is.
an integral part of the cellular substance, and it is evident
that the bacterial cell must synthetically produce this
protein body during its growth from the chemical constituents
of the medium.
When the protein is split up by dilute alkali in absolute
alcohol according to the method described, the poison is
in solution in the alkaline alcohol. The preparation is
filtered and the filtrate neutralized with hydrochloric acid,
avoiding an excess of acid. This throws down the greater
part of both base and acid as sodium chloride, which is
removed by filtration. In this way a solution of the poison
in absolute alcohol is obtained. This is evaported in
vacuo at 40, redissolved in absolute alcohol to remove
traces of sodium chloride, and again evaporated in vacuo
at 40 or less. Evaporation may be done in an open dish,
but the toxicity of the substances is somewhat decreased
when this is done. The poisonous part of the protein
molecule when obtained in this way and powdered, when
there is no water present, forms a dark brown scale which
pulverizes into a lighter brown powder.
It should be clearly understood that we regard this
method of extracting the poisonous group from the protein
molecule as by no means ideal. We know that it is crude
and that much of the poison is destroyed in the process.
In disrupting a protein by our method with dilute alkali
in absolute alcohol, ammonia is given off and the odor of
this gas is apparent even at the end of the third extraction.
An effort was made to discover how much nitrogen was
converted into ammonia in the process. A device was
arranged for conducting the ammonia into standard acid,
and four 10-gram samples of Witte’s peptone were extracted
with 2 per cent, sodium hydrate in absolute alcohol, one
for three hours in a current of air, the others in a current
of hydrogen for two and one-half, eight and one-half, and
nineteen and one-half hours respectively. At the end of
each operation the excess of acid was titrated with deciTHE
CLEAVAGE OF PROTEINS WITH ALKALI 101
normal sodium hydrate, and the percentage of nitrogen
calculated. The relative toxicity of the split products was
determined. In every case ammonia was still being produced
when the process was interrupted. Again, a 10-gram
sample of the poison from egg ablumen was boiled for
fifty-four and one-half hours with 2 per cent, alcoholic
alkali to ascertain if ammonia could be split from the
poison itself. The results of this work are shown in the
following table:
AMMONIA PRODUCED BY CLEAVAGE OF PKOTEIN WITH DILUTE ALKALI IN
ABSOLUTE ALCOHOL.
Per cent, of
Time in Atmos- N given Rate per
Sample. hours.
Witte peptone 3.0
Witte peptone 2,5
Witte peptone
Witte peptone
Poison
8.5
19.5
54.5
phere.
air
H
HH
II
off.
102 PROTEIN POISONS
The brownish toxic powder, varying in shade of color
somewhat with the protein from which it has been obtained,
has a peculiar odor. It is highly hydroscopic, and the
poisonous .portion is freely soluble in water. The solubility
of the whole powder, however, varies with the protein
from which it is obtained, and possibly with the length
of time that it has been exposed to the alkali in the alcohol.
Any portion insoluble in water should be removed by
filtration, and in some instances we have found filtration
through porcelain necessary. Generally the powder dissolves
in water with a slight opalescence easily removed by
filtration through paper. In all cases we have found the
portion insoluble in water free from toxic effect. Aqueous
solutions of the poison are decidedly acid to litmus, the
acidity being due to some organic body and probably
not to the poison itself. On neutralization with sodium
bicarbonate a brownish, non-toxic precipitate is formed.
Prolonged contact with alkali, as we shall see later, lessens
the activity of the poison, and even neutralization has
some effect, which is more marked the longer the preparation
stands. We are inclined to attribute this to the formation
of a salt with the acid poison and the alkali. The
poison is freely soluble in alcohol, more readily than in
water. Alcoholic solutions on long standing deposit small
brownish sediments which we have always found to be
inert. When an alcoholic solution is evaporated, there is
a part of the residue that is insoluble in absolute alcohol.
These portions also are devoid of toxic effect. Alcoholic
solutions have been kept for five years without recognizable
loss in toxicity, and even aqueous solutions decompose
very slowly. The poison is soluble in methyl as well
as in ethyl alcohol. It is insoluble in ether, chloroform,
and petroleum ether. Each of these removes a small
amount of fatty substance, which is non-toxic, but they
do not dissolve an appreciable quantity of the poison.
From its alcoholic solution the poison is precipitated by
ether, but contact with ether decreases its toxicity to such
an extent that this method is not applicable in attempts
at purification.
THE CLEAVAGE OF PROTEINS WITH ALKALI 103
The “crude soluble poison” is soluble in strong mineral
acids, and such solutions remain clear on being boiled and
on dilution with water. However, a few drops of mineral
acid added to an aqueous solution cause a precipitate,
which seems to indicate that the acidity of the aqueous
solution is caused by the presence of some organic acid.
The poison diffuses slowly through collodion sacs both
within the animal body and when suspended in distilled
water. The following experiments bear on this point:
Two hundred milligrams of the crude soluble poison from
the cellular substance of the typhoid bacillus dissolved in
20 c.c. of water was placed in each of two collodion sacs
which were then suspended in distilled water. At the
end of twenty-four hours, the Millon reaction was given
by the dialysate. This was replaced every twenty-four
hours by fresh distilled water, and the dialysis continued
for ninety-six hours. At the end of this time the combined
dialysates were concentrated to dryness, the residues dissolved
in absolute alcohol, filtered, and again evaporated.
The brown, sticky residue, thus obtained, dissolved in
water, was acid in reaction, had the characteristic odor,
and when injected into a guinea-pig, killed in twenty minutes
with typical symptoms, thus showing that the poison does
diffuse through a collodion sac. So slowly, however, does
it diffuse that at the end of ninety-six hours it was not
wholly removed from the sac. In another experiment
one gram of the same poison in 8 c.c. of water was put into
a collodion sac which was introduced into the abdominal
cavity of a medium-sized rabbit. After twelve days, the
animal not being visibly affected, the sac was removed
and found to contain 6 c.c. of a clear fluid which looked
more like blood serum than anything else. Five cubic
centimeters of this injected into the abdomen of a guineapig
had no effect. We conclude from this that the poison
had diffused from the sac, but so slowly that it was disposed
of by the animal’s body without recognizable discomfort.
Notwithstanding the ready solubility of the crude soluble
poison in absolute alcohol, we must regard it as either
104 PROTEIN POISONS
being a protein itself or as being mixed with one or more
proteins. Its aqueous solutions give all the protein color
reactions with the important exception of that of Molisch.
It is worthy of note that the part that separates from
alcoholic solution on long standing is inert and does not
give the protein reactions, while the solution does not
decrease in toxicity. This indicates that the protein is
permanently soluble in absolute alcohol. The Millon
reaction shows most perfectly and persistently whenever
the poison is found. It is generally believed by physiological
chemists that this reaction is given by all benzene
derivatives in which one hydrogen atom has been replaced
by a hydroxyl group, and it is also generally supposed that
tyrosin is the only oxyphenyl compound in the protein
molecule, therefore this reaction is presumed to show the
presence of tyrosin. This is interesting in view of the
fact already stated that gelatin, which contains no tyrosin,
or but little, yields no poison. The fact that the poison
contains no carbohydrate, as shown by its failure to respond
to the Molisch test, an exceedingly delicate test, is, in our
opinion, strong evidence that the cleavage in the protein
molecule induced by dilute alkali in absolute alcohol at
the temperature of 78 follows along structural lines. If
the change were one of simple degradation without chemical
cleavage it wrould be difficult to explain the absolute failure
of the carbohydrate test in the crude soluble poison. It
seems quite evident from our work that in the process the
complex protein molecule is split into several groups, one
of which is the poison and another is a carbohydrate, the
former being freely soluble in absolute alcohol, while the
latter is insoluble in this reagent. It should be stated that
the crude, soluble poison not only fails to respond to the
Molisch test, but it also fails to reduce Fehling’s solution
after prolonged boiling with dilute mineral acid.
The crude soluble poison gives the biuret test beautifully,
therefore we must say that the poison either is itself
a biuret body or is mixed with such a body. As is well
known, the biuret test is regarded as the landmark between
THE CLEAVAGE OF PROTEINS WITH ALKALI 105
proteins and their simpler non-protein disruption products,
and, so long as a disrupted protein continues to give the
biuret test it must still be classed among the proteins. It
will certainly be understood that the pure poison may
not be a protein, but until it is purified sufficiently to fail
to give the biuret test it must be regarded as a protein.
The poison responds nicely to the Adamkiewicz or glyoxylic
acid test. Hopkins and Cole have shown quite
convincingly that this color test depends upon the presence
of tryptophan or indol-amino-propionic acid; therefore, while
we have made no direct search for tryptophan in our poison,
we assume its presence on account of the unequivocal
response to this test.
When the poison is boiled with concentrated hydrochloric
acid to which a drop of concentrated sulphuric acid has
been added, the powder passes into solution and a violet
color results, thus giving Liebermann’s test. At one time
Hofmeister believed this to be a carbohydrate reaction in
which furfurol and the aromatic oxyphenyl radicals take
part, but Cole has shown that this, like the Adamkiewicz
test, also once regarded as a carbohydrate test, is due to
the tryptophan group. We are quite convinced that our
soluble poison contains no carbohydrate, and we regard
the fact that it does respond to the Liebermann test as a
strong confirmation of the error of Hofmeister’s explanation
of this test, and in favor of the explanation given by Cole.
When heated with strong nitric acid the powdered poison
goes into solution, more or less yellow according to the
amount used, and this becomes orange on the addition of
ammonia, thus giving the xanthroproteic test and indicating
the existence of aromatic radicals.
The ordinary test for sulphur in proteins, that of heating
with excess of sodium hydrate in the presence of a small
amount of acetate of lead, is not given by the portion of
the protein split off “by alkali in absolute alcohol. If,
however, a portion of the substance in a test-tube is fused
with metallic sodium and the cooled mass treated with
water, a few drops of a freshly prepared solution of sodium
106 PROTEIN POISONS
nitroprussiate added to a part of the clear filtrate, a beautiful
violet color is produced, indicating the presence of sulphur.
Also, if the other part of the clear filtrate be treated with a
lead acetate solution, lead sulphide is precipitated. If the
solution be acidified before lead acetate is added a faint
but unmistakable odor of hydrogen sulphide is detected.
It is known that sulphur may exist in the protein molecule
in at least two forms, one part being readily split off with
dilute alkali as a sulphide, the other being obtained only
when the disruption of the protein molecule is carried much
farther. It is still a question whether or not both of these
sulphur groups come from cystin. Since the nitroprussiate
reaction is very delicate, no conclusion as to the amount
of sulphur can be drawn from this test, and although a
good precipitate of lead sulphide is formed, the amount of
sulphur in the poison is probably not large, since Leach
failed entirely to find sulphur in the ash of the colon bacillus,
though both the cellular substance and the non-poisonous
portion, as well as the poison, respond to the nitroprussiate
test for sulphur and also give the lead sulphide precipitate
in the clear acidified filtrate from the fused mass.
A solution of this toxic substance is not coagulated by
heat in acid, neutral, or alkaline solution, though, as already
stated, a few drops of a mineral acid added to an aqueous
solution causes the appearance of a considerable precipitate,
which is not soluble on heating or on the further addition
of acid. This precipitate is produced regardless of the
previous removal of the opalescence from the aqueous
solution.
Among the metallic salts, copper sulphate produces no
precipitate and ferric chloride only on heating. Silver
nitrate naturally precipitates any trace of chlorides present,
but after the addition of an excess of ammonia there still
remains a small precipitate. Potassium ferrocyanide gives
a precipitate, also potassium bismuth iodide in acid solution.
Lead acetate, mercuric chloride, and platinum chloride
all produce heavy precipitates. With lead acetate and
mercuric chloride, however, after removal of lead and
THE CLEAVAGE OF PROTEINS WITH ALKALI 107
mercury with hydrogen sulphide from their respective
precipitates and filtrates, the protein reactions are given
by the filtrates, and here also is found the poison in each
case. From 10 to 15 per cent, of the crude poison can be
precipitated by the use of platinum chloride in either water
or alcoholic solution. All attempts to crystallize this
precipitate failed, as only a small part of it is dissolved by
hot water, and the insoluble part is unaffected by any of
the ordinary solvents. The protein reactions are given
by the platinum precipitate, by both soluble and insoluble
parts, but not by the filtrate. The poison is found in the
insoluble part of the precipitate after removal of the platinum
by hydrogen sulphide, its toxicity being markedly
increased. The other parts, after removal of the platinum,
are inert.
The most active products have been obtained by precipitation
from solution in absolute alcohol with alcoholic solutions
of the chlorides of platinum, mercury, and copper and
removal of the base from the precipitate with hydrogen
sulphide. By this method we have obtained a body which
kills guinea-pigs of from 200 to 300 grams’ weight in doses
of 0.5 mg. given intravenously.
From a water solution of the poison, bodies giving protein
reactions may be salted out by the addition of ammonium
sulphate or sodium chloride to saturation, but in neither
case is the separation complete, the filtrates still responding
to the protein color tests after removal of the neutral
salts. In case of salting out with ammonium sulphate, the
solubility of both parts is thereby lessened and the toxicity
diminished, possibly on account of decreased solubility,
though both parts exhibit some poisonous action, and likewise
both show the protein color tests.
Phosphotungstic, phosphomolybdic, and picric acids all
give abundant precipitates. Since these reagents are also
used in the precipitation of alkaloidal bodies, the precipitates
.with phosphomolybdic and phosphotungstic acids were
further examined, the possibility suggesting itself that the
toxic body might be alkaloidal in nature, and that the
108 PROTEIN POISONS
protein part might be entirely separate from the poison.
A sample was precipitated with phosphomolybdic acid
in acid solution, the precipitate removed, washed, and
dissolved in ammoniacal water. This solution was then
shaken with amyl alcohol, but the alcohol was not colored
and the residue obtained on concentration was so slight as
to be practically nothing. Another sample was precipitated
with phosphotungstic acid, the solution being acid in
reaction. The precipitate was allowed to settle, removed
by filtration, washed with acidulated water, decomposed
with a saturated solution of barium hydrate, and the
remaining insoluble part filtered out. So far as possible,
the barium was removed from the filtrate with carbon
dioxide, alternating with concentration, and further addition
of carbon dioxide. The solution was then allowed to
concentrate to dryness, when the residue was dissolved in
absolute alcohol, leaving barium salts behind. On concentrating
the slightly opalescent solution, more barium
salts came down during the process and were filtered out.
The dry residue was taken up in water and ammonium
carbonate used to precipitate the barium that still remained.
After removing the barium carbonate by evaporating on
the water-bath, both carbon dioxide and ammonia were
expelled, the solution again becoming acid. Dryness being
reached, absolute alcohol was once more used, leaving
undissolved a small amount of inorganic material. In this
way the final residue after evaporation of the alcohol was
practically freed from inorganic impurities. Sulphuric
acid no longer gave a barium precipitate in water solution.
The amount obtained by this method was very small and
an exceedingly small part of the original toxic powder.
Since the substance obtained in this way still gave good
Millon’s, biuret and xanthoproteic reactions, it is fair to
say that it was not alkaloidal. The very small amount
obtained by this method given to a guinea-pig intraabdominally
made the animal sick, but did not kill.
Either phosphotungstic acid does not precipitate the toxic
body or else the amount obtained was less than a fatal
dose.
Should the poison consist of an alkaloidal body existing
as a salt in the acid solution, the possibility of extracting
the base with ether or chloroform, after the solution had
been made alkaline with ammonia, is apparent. This
was tried with negative results. To a water solution of
colon poison, acid in reaction, ammonia was added, drop
by drop, to a slightly alkaline reaction, the mixture shaken
with ether, the ether separated and evaporated. The residue
remaining was non-toxic. The ammoniacal water solution
was next shaken with chloroform, the slightly colored
chloroform drawn off and evaporated at low temperature,
leaving a small amount of a dark, thick, semiliquid, which
was not poisonous either as it was or after faintly acidifying
with hydrochloric acid. The water solution remaining
being still poisonous, it is evident that the toxic part is not
an alkaloidal body capable of being extracted directly.
Potassium bismuth iodide in acid solution of the crude
soluble poison produces an abundant precipitate, apparently
more or less soluble in excess, and soluble in ammoniacal
water.
Kowalewsky has shown that uranyl acetate will completely
remove from various albuminous fluids every trace
of protein giving a biuret reaction, while Jacoby and others
have used this reagent for the removal of proteins from
faintly alkaline solutions. Abel and Ford used it to remove
protein from an extract of poisonous fungi. In a slightly
alkaline solution of albumin poison, uranium acetate gave
an abundant precipitate, but not a complete separation,
as both precipitate and filtrate still gave the Millon and
biuret tests, and the filtrate, after removal of excess of
uranium with a solution of di-sodium hydrogen phosphate,
filtration, evaporation, solution in alcohol, and reevaporation,
was still poisonous. In acid solution, the precipitation
was complete, the filtrate no longer giving the protein
reactions.
Freshly prepared metaphosphoric acid also produced
an abundant precipitate, but not a complete separation,
the filtrate showing both Millon and biuret reactions.
110 PROTEIN POISONS
Likewise a heavy precipitate is produced by the use of a
saturated solution of picric acid, but the poison is not in the
precipitate, which gives only a very poor Millon test after
removal of the picric acid, and no biuret. Hofmeister has
given a method for introducing iodine into the molecule
of egg albumen. This was tried with the poison split from
egg albumen. The iodized compound no longer gave either
the Millon or biuret reactions, and while it affected animals
more or less, they did not die, and the symptoms were not
those induced by toxin poisoning. The iodine seemed to
have entered into chemical combination in the poison
molecule, and to have thus changed its characteristics.
The iodized body was freely soluble in absolute alcohol,
and in alkaline water, not in water alone, and was precipitated
by acid water from alcoholic solution, also on acidifying
an alkaline water solution. Though it no longer
responded to the Millon and biuret reactions, a good test
for nitrogen was obtained after fusing with metallic sodium.
An attempt was made to benzoylate the poison by the
Schotten-Baumann method, using albumin poison. Practically
no precipitate was obtained. From the filtrate in a
part soluble in hot alcohol there were obtained shiny, glistening
plates or flat needles which matted together under
suction, and had much the appearance of some of the fatty
acids. These were insoluble in water or very difficultly
so, if at all, difficultly soluble in cold alcohol, readily in hot.
They gave no Millon test, no biuret, no Molisch, and contained
no nitrogen. After recrystallization from alcohol
they melted constantly at 62. Palmitic acid melts at 62
and boils at 339 to 350 (Mulliken). A Merck preparation
of palmitic acid melted at 62 and boiled at about 345 to
350. Our crystals had not yet boiled at 360, though
above 300 there was some decomposition. From the
remainder of the filtrate there was obtained from the part
soluble in cold alcohol a non-crystallizable body, giving
both Millon and biuret tests and containing 9.335 per cent,
of nitrogen, and from the part soluble only in water, likewise
a non-crystalline compound, with 9.66 per cent, nitrogen,
THE CLEAVAGE OF PROTEINS WITH ALKALI 111
and showing both Millon and biuret tests, but not seriously
affecting animals in usual doses.
The nitrogen in a number of the crude poisons has been
determined by Gidley in this laboratory as follows:
PERCENTAGE OF NITROGEN IN PROTEIN POISONS.
Per cent, of N.
Source of poison. in crude poison.
Colon bacillus 13.49
Typhoid bacillus 11.52
Tubercle bacillus 11.00
Pyocyaneus 10.50
Ruber of Kiel 10.495
Subtilis 8.12
Megaterium 8.595
Proteus vulgaris 10.17
Yellow sarcine 6.145
Egg albumen (Leach) 13 . 74
Serum albumin 10.48
Edestin 12.78
Zein 10.69
Witte peptone 11.14
De Chapoteaut peptone …….. 12.735
To study the distribution of the nitrogen, determinations
were made in both the colon and albumin poisons, of the
ammonia nitrogen, the mono-amino, and diamino nitrogen,
by the method already described under cleavage with
dilute mineral acids. The following are the results:
DISTRIBUTION OF NITROGEN IN PROTEIN POISONS.
Total N Mono-
Source of Total of acid Ammonia amino Diamino
poison. poison. extract. N. N. N.
Colon bacillus . 13.49% 10.185% 1.525% 6.472% 1.753%
Egg albumen . 13.74% 11.477% 0.745% 7.999% 1.400%
It will be seen that the greater part of the nitrogen is to
be found in mono-amino combination. From the phosphotungstic
filtrates, from both the albumin and colon poisons,
containing the mono-amino acids, crystalline bodies were
obtained. Judged by the strong Millon test, tyrosin was
112 PROTEIN POISONS
undoubtedly present, but the crystalline masses were
largely leucin, and no tyrosin was obtained in purified
form. From the crude crystals, after many and repeated
crystallizations, what was thought to be leucin was obtained
pure, melting at 264 to 265 uncorrected, or 269.42 to
270.46 corrected. The crystals were thin plates characteristically
grouped, and sublimed readily. From another
5 per cent, sulphuric acid extract of albumin poison was
obtained a large mass of crystals in characteristic tyrosinlike
sheaves, and giving a deep Millon reaction. These
were undoubtedly tyrosin, though at the time no meltingpoint
was taken.
Properties of the Haptophor or Non-poisonous Group. Leach1
has investigated this split product with the following
general results: After cleavage of the protein with alkaline
alcohol, the haptophor remains undissolved. It is collected
on a filter, then transferred to Soxhlets, and for some days
extracted with 95 per cent, alcohol. This is for the purpose
of removing as thoroughly as possible the alkali which it
has absorbed from the alkaline alcohol. This cannot,
however, be wholly washed out by this method, and it is
possible that in part it is held chemically. After this
extraction the substance is easily reduced to a fine brownish
powder. On burning it puffs up, gives off the characteristic
odor of nitrogenous compounds, and leaves a copious ash
containing phosphate. The solubility of the haptophors
from different proteins differs widely; that from egg-white
is wholly soluble in water, while that from the cellular
substance of the tubercle bacillus is only sparingly soluble.
However, it is only the part soluble in water from any of
these haptophors that is of special interest. The studies of
Leach, referred to, were made with the non-poisonous
portion of the colon bacillus. This is mainly soluble in
water, giving an opalescent solution from which a lightcolored
sediment is deposited on standing, leaving a clear,
golden brown solution. The sediment is not soluble in
1 Jour. Biolog. Chem., 1907, iii, 443.
THE CLEAVAGE OF PROTEINS WITH ALKALI 113
either dilute alkali or acid in the cold, but is soluble in
alkali on boiling. The clear, aqueous solution of the haptophor
is alkaline from sodium hydrate held either mechanically
or chemically; it is precipitated by mineral acids and
by alcohol. It responds to the biuret, xanthoproteic,
Millon, and Adamkiewicz tests. Millon’s test is not very
satisfactory, and in some samples has failed altogether,
even after care has been exercised in neutralizing the alkali.
It is quite evident that the substance or substances in the
protein molecule to which the Millon test is due are for
the most part found in the toxophor group. However,
the readiness of response to this test varies greatly in the
different haptophors. The haptophor substance does not
reduce Fehling’s solution directly, but does so readily and
abundantly after prolonged boiling with dilute hydrochloric
acid. The presence of carbohydrate in the haptophor
has already been discussed (page 70). Tests with
a-naphthol, phloroglucin, and orcin give positive results.
Ammonium molybdate gives an organic precipitate, but
no evidence of free phosphoric acid. The preliminary tests
show the presence of protein, nucleic, and carbohydrate
groups. Comparing these results with those obtained in
the study of the toxophor, the following statements may
be formulated: (1) The toxophor is freely soluble in absolute
alcohol, the haptophor is insoluble in this menstruum.
(2) The toxophor contains no carbohydrate, all of which is
found in the haptophor. (3) The toxophor freely responds
to the Millon test, while the haptophor does so slightly and
in some instances not at all. (4) The toxophor contains no
phosphorus, or but little of this element, while the haptophor
is rich in phosphorus. (5) The toxophor from different
proteins seems to be the same, possibly with unrecognizable
differences in chemical structure, while the haptophor of
each protein differs from that from all other proteins.
Leach1
gives the following table showing the percentages
of ash, nitrogen, and phosphorus in the haptophor of the
colon bacillus:
1 Loc. cit.
ash.
THE CLEAVAGE OF PROTEINS WITH ALKALI 115
nucleic acid and the nucleates are the only nucleo compounds
in which the ratios are at all comparable with
those given in the preceding table. Nuclein contains a
little less phosphorus than any of these preparations from
the germ, while other nucleo compounds are much richer
in nitrogen and poorer in phosphorus. It is perhaps worthy
of mention that contact with mineral acid apparently
breaks up the nucleic acid, the phosphoric acid going into
solution; thus, preparation A gives evidence of phosphorus
in inorganic combination, while G does not.”
Substance.
116 PROTEIN POISONS
Leach split up edestin, casein, egg-white, and colon
cellular substance with alkaline alcohol. The insoluble
part of each gave the various protein color tests, Millon’s
reaction less satisfactorily than the others. On stirring
with water, the edestin preparation was entirely soluble,
there was a slight flocculence with the casein preparation,
the others were mainly but not wholly soluble. Addition of
a little sodium hydroxide increases the solubility. Mineral
acids give precipitates with the casein and egg preparations.
The most marked difference was found on testing for
carbohydrates. As edestin contains no carbohydrate, its
preparation showed no evidence of such a group. Although
casein is said to contain no carbohydrate, it has been found
to respond to the Molisch test, and so does its haptophor.
As was to be expected, the egg preparation gives evidence
of hexose and not pentose. The lead sulphide reaction
shows the presence of loosely combined sulphur in the
preparations from egg and edestin, not in the ones from
casein and the colon bacillus.
Samples of the haptophor of egg-white were stirred with
water, filtered, and attempts made to separate protein and
carbohydrate in the filtrate by means of uranium acetate.
The acetate was added both with and without sufficient
alkali to keep the solution alkaline. A copious precipitate
resulted in both cases and this was filtered out with some
difficulty. The slight excess of uranium was removed
from the filtrate by the addition of sodium phosphate.
The filtrate gave evidence of carbohydrate, but the separation
was not sufficiently sharp, and that method was
abandoned. Acidifying until there was a slight permanent
precipitate, the addition of either ethyl or methyl alcohol
cleared the solution. Phosphotungstic acid precipitated
both protein and carbohydrate. In short, no method was
found that would remove the protein from the solution and
leave the carbohydrate. It is perhaps a legitimate inference
that the combination of the two is a chemical one.
Samples were subjected to hydrolysis and titrated with
Fehling’s solution. The proteins and possibly other bodies
THE CLEAVAGE OF PROTEINS WITH ALKALI 117
present interfered with the reaction, but by adding the
solution all or nearly all at once it was possible to obtain
comparative results. Experiments with the haptophor of
the colon bacillus had shown that the maximum reduction
was obtained by boiling for two and one-half hours with
2.5 per cent, hydrochloric acid (see p. 70).
Three grams of the haptophor of egg-white was mixed
with 200 c.c. of water, and 20 c.c. of 25 per cent, hydrochloric
acid. A second sample was prepared in the same
way except that it was filtered before adding the acid.
Both were boiled with reflux condenser. After boiling half
an hour and then at intervals of three hours, aliquot parts
were removed, neutralized, titrated with Fehling’s solution,
and the amount of reducing substance calculated. Other
samples were hydrolyzed with sulphuric acid, with less
satisfactory results. These preliminary experiments indicated
that the reducing substance is all present in the
portion soluble in water, and that the maximum yield,
which if calculated as dextrose, is about 9 per cent., is
obtained by boiling from ten to twelve hours, and until the
mixture no longer gives the biuret test.
Accordingly, 25 grams of the egg-white haptophor was
shaken for two hours on a shaker with ten times its weight
of water, filtered, 200 c.c. more of water added, the solution
neutralized with hydrochloric acid, then 50 c.c. of 25 per
cent, hydrochloric acid added, thus making approximately
a 5 per cent, solution of material in 2.5 per cent. acid. This
was boiled with a reflux condenser for ten or twelve hours,
until the solution no longer gave the biuret test. It was
then filtered, leaving very little on the filter. The clear,
red-brown filtrate was cooled, neutralized with sodium
hydroxide, and benzolated by the Schotten-Baumann
method. The mixture became very warm, but was cooled
by surrounding the flasks with pounded ice and salt. When
the reaction ceased, the compound settled nicely, and was
filtered by suction after standing two or three hours. The
precipitate was washed with water containing a little
ammonia, and treated with boiling water, in which a large
118 PROTEIN POISONS
portion was freely soluble. On cooling and concentrating
the alcoholic solution, a fine yield of crystals was obtained.
The crystals from several samples were united and recrystallized
from hot absolute alcohol until the solution was
clear and colorless. Macroscopic bundles of needles were
thus obtained, showing very characteristic grouping. They
were washed in alcohol and in ether, dried upon porous
plates, the operations being repeated until samples from
two recrystallizations melted side by side within 1 or 1.5.
The crystals are pure white, readily soluble in benzol,
chloroform, and in glacial acetic acid as well as in alcohol,
and melt at 203. When boiled with sodium hydroxide,
ammonia is given off; after removing benzoic acid by boiling
with hydrochloric acid, the resulting product reduces
Here in Houston the spraying was really bad yesterday. It was really windy and seemed like a cloudless day until about noon. Then the spraying started. The weather says mostly cloudy but satellite images show a pretty clean spot over northwest Houston. Like maybe they scrub the images before they go up? On the walk home from the bus stop and maybe a total of 20 minutes outside talking to neighbors to point out the trails, everyone of my kids has something today. My youngest is 3 and complained of his ears hurting him all night long and he rarely if ever has had ear infections. My 5 year old woke up with a bunch of crust on one eye, what is that?! And my 7 year old just developed a cough. I am noticing that my older two with allergies seem to be the most affected on days like this, but this was really quick. It was so heavy that there was a rainbow like halo around the sun and some of the clouds seemed to be lighting up with a rainbow effect as well. So so sad….
Start wearing masks they use when spray painting, people will ask you why you are wearing it. This gives you an excuse to educate people without being pushy and people shall except your explanation sooner. You can also refer them to a website to seek for themselves if they do not believe you.
Just wanted to report the largest chem-carpet I’ve ever seen here in Los Angeles. It started on March 15th (5 days ago), earlier last week up until the 15th we’ve had crystal clear skies – but every day since the 15th there have been massive sprays in all directions as well as evenly spaced rakes through the skies, several planes at once in all areas of the sky; every day. The sun emulates the strangest glow through the silvery “clouds”…it’s bizarre, guys. I’ve never been this website, but I found it through a random search to see if anyone else was talking about it. I mean, this is a THICK carpet like nothing Ive ever seen. And this past week, everyone I know including myself has fallen sick. I’ve had a flu, coughing up yellow phlegm every day. Sick as a dog, all my friends too. This is baaaad. I was looking up at it today, you can hardly see streaks anymore it’s just all fake clouds – silver haze. Where there are breaks in the clouds, there are new planes and chemtrails. Everytime I look up there’s another plane. They’re freaking everywhere. 🙁
It sounds like Los Angeles is getting hit hard, Bianca. I’m sorry to hear it’s so bad there. Thanks for sharing this so others can know that they’re not alone. There are some things you can do to build up your immune system and not get sick so much. Look into those and vigilantly protect yourself. Some tips are on our ‘health’ page. Diatomaceous earth detoxifies metals and other things from your system. A netti pot cleanse w/ a drop of tea tree oil in it daily, seems to help to ward off the viruses. I inhale steam from colloidal silver when I feel a virus coming on and it seems to stop it. Stay away from all genetically modified, and processed foods. etc… Nutritional information can be found on naturalnews.com. We need to wake people up to this insanity, Bianca. They’re all quite obliviously asleep, as if under a spell. Programmed and conditioned from birth. Some of us manage to break free and see the truth. We need to help others do the same. Keep looking up, and take heart! You’re not alone. Thanks for writing.
Bianca, I’m in LA and have been seeing the same thing. Even from a high point in Marina del Rey I look out and see this horrible grey/brown haze over all of the Westside. And there are constantly more planes that break through the wisps of chemtrails hours old.
What can we do to raise awareness here?
Yesterday was the worst spraying we have ever witnessed. I live in SE TN on the NW GA border. We saw what my daughter described as “rainbow portals”. We counted 6 planes at one time and at least 60 trails over a 15 mile traveling distance. This went on from sun up to sun down.
Here is the kicker, today I went to pick up some supplies. I went to several different places. At my first stop a clerk said to another “I am so tired” and the co-worker replied, “me too, I feel so sleepy today”. There was my door! I jotted some info down for them to look up. Next stop I see a mom and a 10 yr old or so walking by and the little girls says “Mom! I am soooo tired”. Mom replied, “I know, me too honey”. This is really getting serious. I’m glad I found your site tonight! This is going to need a huge collective of people to be very organized and act quickly. It was shocking to realize how long they have been doing this.. I only found out about it a year ago or so when I started investigating the pains and symptoms I was having from this disgusting genocide!
My gosh, no one wants to hear this. I have tried to tell people, put the videos on Facebook, but everybody is too asleep! They are so wrapped up in the financial chaos.Or they make fun of this, as well as the current radiation problem. I want to make the most of my time and this information. What is the best approach? Most people are not willing to believe it.
Don’t give up, we have to keep trying to get the word out. These demons will not win! I have found an approach that is working. Instead of trying to tell them all about it, listen to what they are saying. As soon as they say something like “I have been so achy lately” or “I feel so sleepy all the time” (a couple of the side affects from this evil spraying), then that is your time to ask “Have you noticed those white lines in the sky?”. If they say yes just give them a link to info or a video. If they say no, tell them to start watching the sky and see if they notice those white lines that “don’t go away”. Just remember, the less you say the more curious they are likely to be. Print up some business cards with just info on them to hand out. Stay strong and vigilant everyone…evil NEVER wins in the end!
I’ve has the same experience Marie. I point out the spraying to people and actually get laughed at!
Some are receptive but the majority are in some sort of trance with their i-pods.
I notice that all the time too Al.
i’m in kamloops b.c. the snow arrived here overnight and looks like the pebbling you see on a curling ice surface. this was preceded by an all out assault of chemtrails and howling, swirling cold blasts of winter over the last 2 days.
I am in Edmonton, Alberta, and we had the warmest February on record. And quite a reversal in March, and week of snow, little bit more every night, strange sticky snow some of the time, and unusual cold spell following. And yesterday, while shovelling snow under overcast skies, heard at least 6 low-flying jets in short order – we are miles and miles from the airport.
Would be happy to contribute to a bill board campaign to alert the public. Also ; try anti war web site. Justine Raimondo is one of the most articulate and persuasive minds in this country. He would probably help. Our senators and congressmen are loyal only to their large contributors so forget about them. This sheisse is falling on phoenix arizona every day now. We think it may make sense to move; but where?.
Wiley….Nice to see such enthusiasm!:) Got our cards and DVDs. Been handing out to those who are receptive.
Have taken hundreds of photos the last few days. Lots of trails and strange clouds here in Northwest CA. It looks like all the clouds have been sprayed. The sky has a very surreal look to it, and is the wrong color.
It is also strangely dry, desicated.
You can see changes from last Spring. Looks like they may have taken over natural weather systems.
Scarey… but I think I would rather know. So nice to have all this information here on “The Geo-Watch” to access.
I agree with you wholeheartedly Wiley. Keep up the good work!
Thanks so much for your hard work and amazing info, Dane. On Coast to Coast a caller asked what foods contain the least amount of aluminum. You said the larger organisms–trees. But you show a diagram in a previous interview that plants die off with aluminum particulates in the soil. Can you please explain? I’m handing out your cards, displaying your bumper sticker, etc…
I notified Earthjustice.org yesterday, and told them about this web site, and asked them to send a system wide alert to all members of Earthjustice@.
Earthjustice@-for those of you who might not know-, is the lawyer for most environmental groups, such as Sierra Club, etc. Their motto is : ” Because the Earth needs a good lawyer. ”
I would urge all who read this message, to go to http://www.earthjustice.org. and become a member, and reguest that they take on this vital issue. The only way we are going to win this war against insanity, is to get all the help we can in this vital battle !
Nothing else matters really if geoengineering doesn’t stop. I am doing everything I can in little NZ to stop these crimes. Thanks for all you do again Dane
I’m aware – concerned – informing – as rapidly as possible and contacting senators from Texas ! Everyone hurry and help ! Order your cards ASAP !!! For our children this is an emergency !
I would love some cards and bumper stickers. I am trying to make people aware. I’ve signed every petition I can find. Calling senator now